Minister for Health, Stephen Donnelly TD has welcomed the announcement by the European Commission that it has authorised the use of the Pfizer/BioNTech COVID-19 vaccine.
This means that roll-out of the vaccines can begin across the European Union.
Minister Donnelly said: “After a very difficult year, it is great to be moving into 2021 with the wonderful news that the first COVID-19 vaccine has been approved for use in the European Union. Our priority was to ensure that any COVID vaccine administered in Ireland meets all of the rigorous safety and efficacy requirements of the EMA, and so this is a huge achievement by our medical and scientific communities.
“Within the coming days, we will begin administering this vaccine in Ireland. We have detailed plans in place for this roll-out through the National COVID-19 Vaccination Strategy. The most vulnerable will be prioritised first and over time, these vaccines will allow us to re-open our society and economy. In the meantime, I am asking everyone to continue to follow the public health guidance. COVID-19 is still with us, and it is still deadly. We all know the right actions – wash your hands, wear a face covering, maintain a two-metre social distance and remember that every contact counts.”
The European Medicines Agency’s human medicines committee (CHMP) and its experts have been working intensively to evaluate data submitted by BioNTech and Pfizer. Following that work, today the EMA has recommended granting a conditional marketing authorisation for the Pfizer BioNTech vaccine. This was granted by the European Commission this evening.
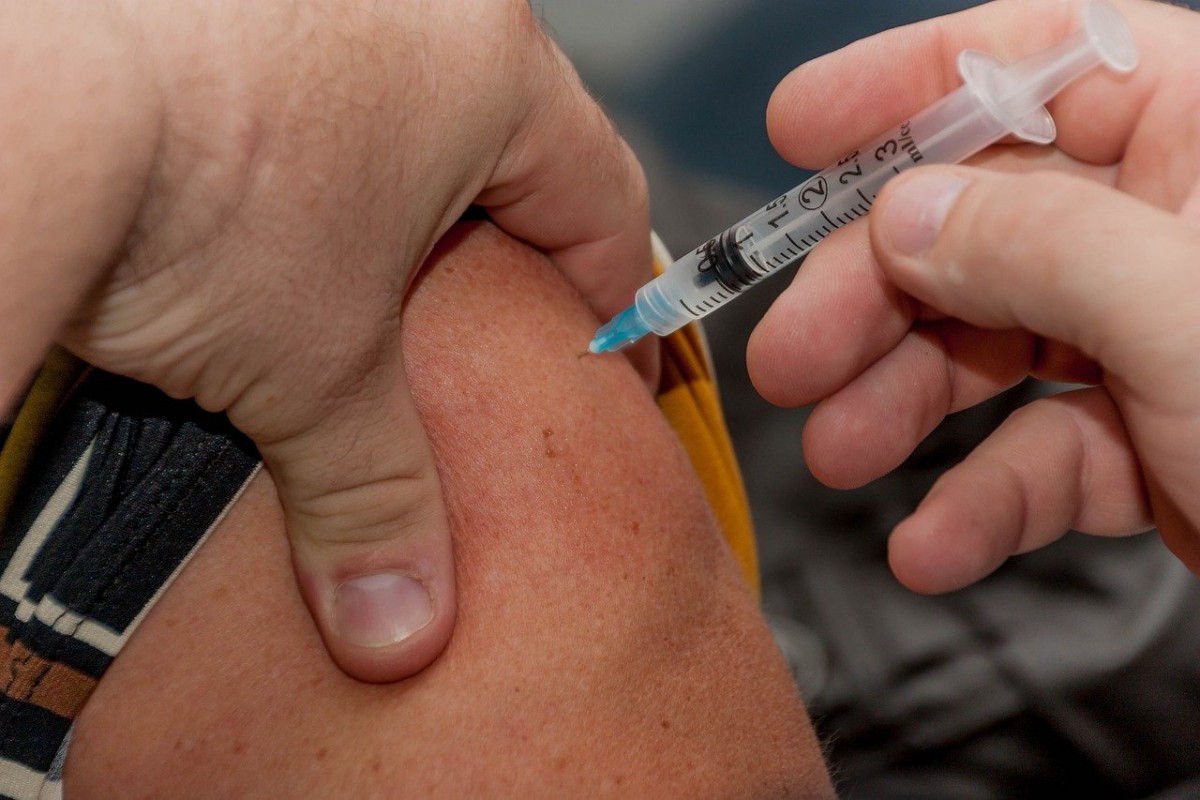
The vaccines will be made available free of charge in Ireland and have been authorised for use for those above the age 16. Comirnaty is given as two injections into the arm, at least 21 days apart.
The vaccines will be rolled out in three phases – the initial roll out is expected to begin before the end of the year. This will be followed by a mass ramp-up as more vaccines become available, and then open access.
The highest priority groups, those over the age of 65 living in long-term care facilities and frontline healthcare workers in direct patient contact, will receive the vaccine first.
Vaccines will be administered from long-term care facilities, hospitals, mass vaccination clinics, GP surgeries and community pharmacies. This will be done by qualified and trained healthcare workers, including hospital doctors, community medical officers, nurses, GPs and pharmacists.
Minister Donnelly said: “I would like to take a moment to thank the health care professionals who will now move to administer this vaccine safely. They have worked selflessly all year to protect us from the worst impacts of COVID-19 and will continue to do so as the vaccine becomes available. We all have a role to play in supporting their heroic efforts by trying our best to avoid catching and transmitting this virus over the coming festive period.”